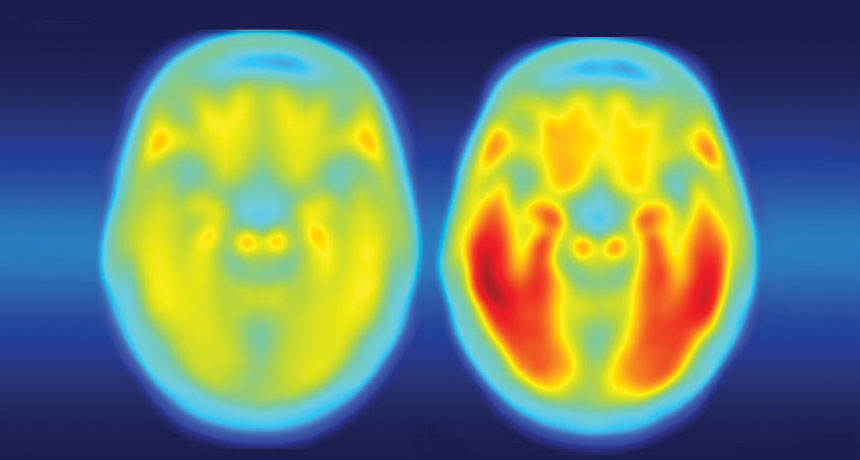
Chronic flu patients could be an early warning system for future outbreaks
People with weakened immune systems might help scientists get a jump on the flu virus.
Some flu virus mutations popped up again and again in cancer patients with long-term infections, researchers report June 27 in eLife. And some of those mutations were the same as ones found in flu viruses circulating around the world a few years later, evolutionary virologist Jesse Bloom of the Fred Hutchinson Cancer Research Center in Seattle and colleagues discovered. The findings may eventually help vaccine developers predict flu strain evolution.
“You can’t predict what’s going to happen next year,” — at least not yet, Bloom says. But monitoring infections in many people may indicate which parts of the virus are most likely to change in the future.
Most people who catch the flu get over it in about a week. Previous studies have suggested that the virus doesn’t change much within one person. It must pass through tens to hundreds of people to build up enough mutations to give it an advantage over other flu viruses, Bloom says. That makes predicting flu evolution tricky.
A coffee shop conversation alerted Bloom to a potential treasure: multiple nasal wash samples from four cancer patients who had had the flu for months in 2006 and 2007. Part of their cancer treatments had weakened all four people’s immune systems, making it hard for them to fight off the infections.
Evolutionary biologist Katherine Xue in Bloom’s lab and colleagues examined genetic material from the nasal washes, identifying mutations present in at least 5 percent of flu viruses in each person. The team tracked competition between virus variants within each person over time and compared virus evolution patterns among the patients.
Nine flu mutations popped up in at least two separate patients. Of those, five were in the virus’s hemagglutinin gene. That gene encodes a sugar-studded protein on the virus’s outer surface that helps the virus stick to and invade human cells. The immune system commonly makes antibodies against hemagglutinin that foil the strain’s ability to infect the host again. As a result, the virus has to mutate so that the protein will be different enough to evade the immune system.
Four amino acids of the hemagglutinin protein were frequently changed by mutations in the cancer patients’ viruses and popped up years later in flu strains worldwide, too. Those amino acids are the 138th, 193rd, 223rd and 225th links in the chain of amino acids that make up the hemagglutinin protein.
In some cases, the mutations produced the same amino acid change in both the cancer patients’ and the global virus strains circulating after 2010. For instance, the amino acid valine was altered to isoleucine at position 223. That happened in two cancer patients in 2006 and 2007. After about 2012, nearly all viruses circulating worldwide had the same change.
In another case, those same two cancer patients’ viruses had tyrosine at position 193, but globally circulating viruses had either phenylalanine or serine at that position, the researchers found. Those results indicate that at some spots in the protein, particular changes are important, but other positions are more malleable.
Within patients, viruses carrying different amino acids seemed to directly compete against each other; as one became more frequent, the other was reduced in abundance. That’s the same sort of pattern researchers observe at the global level. Knowing which mutations commonly win competitions in immune-compromised patients may give a preview of winners in the global flu fight.
Not all of the flu mutations that arose in the cancer patients were later found in the general population, says infectious disease biologist Katia Koelle of Duke University. For instance, a mutation called L427F (changing leucine at position 427 to phenylalanine) was found in more than 75 percent of flu viruses in three of the cancer patients, but it was never seen in flu viruses circulating globally. That mutation might give flu viruses some advantage within a person, but might not be efficient at spreading from person-to-person, Koelle says. Studies that compare flu alterations in multiple people won’t immediately tell researchers how to design vaccines, she says, but could point to parts of the virus for further investigation.
Xue and Bloom say they would like to repeat the study, perhaps this time in very young children and elderly people — two groups that also have weaker immune systems than most adults.